Covaxin who approval news today ~ Find Covaxin Who Approval Latest News Videos Pictures on Covaxin Who Approval and see latest updates news information from NDTVCOM. After the WHO granted an emergency use listing to Covaxin the United States will allow travellers vaccinated with Bharat Biotechs Covaxin to enter the country from November 8.
as we know it recently has been searched by users around us, perhaps one of you personally. Individuals are now accustomed to using the internet in gadgets to see image and video information for inspiration, and according to the title of the article I will discuss about Covaxin Who Approval News Today Even as Bharat Biotechs Covaxin got emergency approval from the WHO bringing relief to Indians on the eve of Diwali the agencys chief scientist Soumya Swaminathan has said that this will help speed up Covaxin Approval for children.
If you are looking for Covaxin Who Approval News Today you've arrived at the perfect place. We ve got 20 graphics about covaxin who approval news today adding pictures, pictures, photos, backgrounds, and more. In these page, we additionally provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
Covaxin who approval news today
Collection of Covaxin who approval news today ~ Speaking to NDTV Swaminathan said In this case it should be much faster. Speaking to NDTV Swaminathan said In this case it should be much faster. Speaking to NDTV Swaminathan said In this case it should be much faster. Speaking to NDTV Swaminathan said In this case it should be much faster. The approval of Covaxin for emergency use listing will amount to recognition and help pave the way for people who have taken the dose to qualify for vaccine mandates in other countries. The approval of Covaxin for emergency use listing will amount to recognition and help pave the way for people who have taken the dose to qualify for vaccine mandates in other countries. The approval of Covaxin for emergency use listing will amount to recognition and help pave the way for people who have taken the dose to qualify for vaccine mandates in other countries. The approval of Covaxin for emergency use listing will amount to recognition and help pave the way for people who have taken the dose to qualify for vaccine mandates in other countries. Following the WHOs EUL Listing approval Bharat Biotech MD Dr Krishna Ella said in a video Today Covaxin got EUL from WHO. Following the WHOs EUL Listing approval Bharat Biotech MD Dr Krishna Ella said in a video Today Covaxin got EUL from WHO. Following the WHOs EUL Listing approval Bharat Biotech MD Dr Krishna Ella said in a video Today Covaxin got EUL from WHO. Following the WHOs EUL Listing approval Bharat Biotech MD Dr Krishna Ella said in a video Today Covaxin got EUL from WHO.
The approval given by the World Health Organization WHO to Covaxin has opened up the indigenous vaccine for worldwide use Indian Council of. The approval given by the World Health Organization WHO to Covaxin has opened up the indigenous vaccine for worldwide use Indian Council of. The approval given by the World Health Organization WHO to Covaxin has opened up the indigenous vaccine for worldwide use Indian Council of. The approval given by the World Health Organization WHO to Covaxin has opened up the indigenous vaccine for worldwide use Indian Council of. Explore more on Covaxin Who Approval. Explore more on Covaxin Who Approval. Explore more on Covaxin Who Approval. Explore more on Covaxin Who Approval. Covaxin has been approved by many countries around the world including Australia Greece and Sri Lanka among others. Covaxin has been approved by many countries around the world including Australia Greece and Sri Lanka among others. Covaxin has been approved by many countries around the world including Australia Greece and Sri Lanka among others. Covaxin has been approved by many countries around the world including Australia Greece and Sri Lanka among others.
Around 10 of the 107 billion doses. Around 10 of the 107 billion doses. Around 10 of the 107 billion doses. Around 10 of the 107 billion doses. In what can be touted as a win for India the Technical Advisory Group of WHO which met today gave its nod for the Emergency Use Listing status for Bharat Biotechs Covaxin. In what can be touted as a win for India the Technical Advisory Group of WHO which met today gave its nod for the Emergency Use Listing status for Bharat Biotechs Covaxin. In what can be touted as a win for India the Technical Advisory Group of WHO which met today gave its nod for the Emergency Use Listing status for Bharat Biotechs Covaxin. In what can be touted as a win for India the Technical Advisory Group of WHO which met today gave its nod for the Emergency Use Listing status for Bharat Biotechs Covaxin. The World Health Organisations panel had last week sought additional clarifications from the Hyderabad-based pharma firm. The World Health Organisations panel had last week sought additional clarifications from the Hyderabad-based pharma firm. The World Health Organisations panel had last week sought additional clarifications from the Hyderabad-based pharma firm. The World Health Organisations panel had last week sought additional clarifications from the Hyderabad-based pharma firm.
Around 10 of the 107 billion doses administered so far in the country are of Covaxin. Around 10 of the 107 billion doses administered so far in the country are of Covaxin. Around 10 of the 107 billion doses administered so far in the country are of Covaxin. Around 10 of the 107 billion doses administered so far in the country are of Covaxin. Biocon Chairperson Kiran Mazumdar Shaw on Wednesday November 3 told India Today TV The fact that Covaxin has been approved by WHO is good validation for Indian innovation She said it. Biocon Chairperson Kiran Mazumdar Shaw on Wednesday November 3 told India Today TV The fact that Covaxin has been approved by WHO is good validation for Indian innovation She said it. Biocon Chairperson Kiran Mazumdar Shaw on Wednesday November 3 told India Today TV The fact that Covaxin has been approved by WHO is good validation for Indian innovation She said it. Biocon Chairperson Kiran Mazumdar Shaw on Wednesday November 3 told India Today TV The fact that Covaxin has been approved by WHO is good validation for Indian innovation She said it. The WHO today issued an emergency use listing EUL for Covaxin adding to a growing portfolio of vaccines validated by the UN health agency for the prevention of. The WHO today issued an emergency use listing EUL for Covaxin adding to a growing portfolio of vaccines validated by the UN health agency for the prevention of. The WHO today issued an emergency use listing EUL for Covaxin adding to a growing portfolio of vaccines validated by the UN health agency for the prevention of. The WHO today issued an emergency use listing EUL for Covaxin adding to a growing portfolio of vaccines validated by the UN health agency for the prevention of.
The World Health Organisation WHO on Wednesday approved India-made Covid-19 vaccine developed by Bharat Biotech. The World Health Organisation WHO on Wednesday approved India-made Covid-19 vaccine developed by Bharat Biotech. The World Health Organisation WHO on Wednesday approved India-made Covid-19 vaccine developed by Bharat Biotech. The World Health Organisation WHO on Wednesday approved India-made Covid-19 vaccine developed by Bharat Biotech. 4th November 2021 0034 IST Covaxin Gets WHO Nod. 4th November 2021 0034 IST Covaxin Gets WHO Nod. 4th November 2021 0034 IST Covaxin Gets WHO Nod. 4th November 2021 0034 IST Covaxin Gets WHO Nod. Covaxin was approved for emergency use in India on January 3. Covaxin was approved for emergency use in India on January 3. Covaxin was approved for emergency use in India on January 3. Covaxin was approved for emergency use in India on January 3.
The final approval has come after a meeting of the global health bodys technical advisory group today Nov. The final approval has come after a meeting of the global health bodys technical advisory group today Nov. The final approval has come after a meeting of the global health bodys technical advisory group today Nov. The final approval has come after a meeting of the global health bodys technical advisory group today Nov. 3 following a thorough risk-benefit analysis of adding Covaxin to the global vaccine. 3 following a thorough risk-benefit analysis of adding Covaxin to the global vaccine. 3 following a thorough risk-benefit analysis of adding Covaxin to the global vaccine. 3 following a thorough risk-benefit analysis of adding Covaxin to the global vaccine. Its a momentous occasion for Indian innovation entrepreneurship. Its a momentous occasion for Indian innovation entrepreneurship. Its a momentous occasion for Indian innovation entrepreneurship. Its a momentous occasion for Indian innovation entrepreneurship.
The technical advisory group which gives licence to a vaccine for its emergency use listing EUL had asked Bharat Biotech on October 26 for additional data. The technical advisory group which gives licence to a vaccine for its emergency use listing EUL had asked Bharat Biotech on October 26 for additional data. The technical advisory group which gives licence to a vaccine for its emergency use listing EUL had asked Bharat Biotech on October 26 for additional data. The technical advisory group which gives licence to a vaccine for its emergency use listing EUL had asked Bharat Biotech on October 26 for additional data. Covaxin is made by Hyderabad-based Bharat Biotech FileHighlightsCovaxin has been cleared for use in all age groups 18 However no recommendation News National. Covaxin is made by Hyderabad-based Bharat Biotech FileHighlightsCovaxin has been cleared for use in all age groups 18 However no recommendation News National. Covaxin is made by Hyderabad-based Bharat Biotech FileHighlightsCovaxin has been cleared for use in all age groups 18 However no recommendation News National. Covaxin is made by Hyderabad-based Bharat Biotech FileHighlightsCovaxin has been cleared for use in all age groups 18 However no recommendation News National. The WHO on Wednesday granted approval for Emergency Use Listing EUL for Bharat Biotechs COVID-19 vaccine Covaxin. The WHO on Wednesday granted approval for Emergency Use Listing EUL for Bharat Biotechs COVID-19 vaccine Covaxin. The WHO on Wednesday granted approval for Emergency Use Listing EUL for Bharat Biotechs COVID-19 vaccine Covaxin. The WHO on Wednesday granted approval for Emergency Use Listing EUL for Bharat Biotechs COVID-19 vaccine Covaxin.
File Photo File Photo Australia Monday recognised Indias Covaxin for the purpose of travel to the country as it eased curbs on international travel adding to the list of many other nations which have given their nod to Bharat Biotechs Covid-19 vaccine. File Photo File Photo Australia Monday recognised Indias Covaxin for the purpose of travel to the country as it eased curbs on international travel adding to the list of many other nations which have given their nod to Bharat Biotechs Covid-19 vaccine. File Photo File Photo Australia Monday recognised Indias Covaxin for the purpose of travel to the country as it eased curbs on international travel adding to the list of many other nations which have given their nod to Bharat Biotechs Covid-19 vaccine. File Photo File Photo Australia Monday recognised Indias Covaxin for the purpose of travel to the country as it eased curbs on international travel adding to the list of many other nations which have given their nod to Bharat Biotechs Covid-19 vaccine. The Technical Advisory Group of WHO which met today has recommended the Emergency Use Listing status for Bharat Biotechs Covaxin - a decision that will ease international travel and the export of the indigenous vaccine. The Technical Advisory Group of WHO which met today has recommended the Emergency Use Listing status for Bharat Biotechs Covaxin - a decision that will ease international travel and the export of the indigenous vaccine. The Technical Advisory Group of WHO which met today has recommended the Emergency Use Listing status for Bharat Biotechs Covaxin - a decision that will ease international travel and the export of the indigenous vaccine. The Technical Advisory Group of WHO which met today has recommended the Emergency Use Listing status for Bharat Biotechs Covaxin - a decision that will ease international travel and the export of the indigenous vaccine. Travellers who have taken both doses of Bharat Biotechs Covid-19 vaccine Covaxin will be allowed to enter the US from November 8 new agency ANI reported. Travellers who have taken both doses of Bharat Biotechs Covid-19 vaccine Covaxin will be allowed to enter the US from November 8 new agency ANI reported. Travellers who have taken both doses of Bharat Biotechs Covid-19 vaccine Covaxin will be allowed to enter the US from November 8 new agency ANI reported. Travellers who have taken both doses of Bharat Biotechs Covid-19 vaccine Covaxin will be allowed to enter the US from November 8 new agency ANI reported.
The advisory committee is scheduled to meet today and the approval for Covaxin is expected to be announced after the meeting. The advisory committee is scheduled to meet today and the approval for Covaxin is expected to be announced after the meeting. The advisory committee is scheduled to meet today and the approval for Covaxin is expected to be announced after the meeting. The advisory committee is scheduled to meet today and the approval for Covaxin is expected to be announced after the meeting. Yogi Terms Move As Global Approval For PM Modis Aatmanirbhar Bharat With the World Health Organisation WHO approving Bharat Biotechs COVID-19 vaccine Covaxin for emergency use Yogi. Yogi Terms Move As Global Approval For PM Modis Aatmanirbhar Bharat With the World Health Organisation WHO approving Bharat Biotechs COVID-19 vaccine Covaxin for emergency use Yogi. Yogi Terms Move As Global Approval For PM Modis Aatmanirbhar Bharat With the World Health Organisation WHO approving Bharat Biotechs COVID-19 vaccine Covaxin for emergency use Yogi. Yogi Terms Move As Global Approval For PM Modis Aatmanirbhar Bharat With the World Health Organisation WHO approving Bharat Biotechs COVID-19 vaccine Covaxin for emergency use Yogi. WHOs approval for Covaxin will facilitate process of India helping. WHOs approval for Covaxin will facilitate process of India helping. WHOs approval for Covaxin will facilitate process of India helping. WHOs approval for Covaxin will facilitate process of India helping.
Yogi terms move as global approval for PM Modis Aatmanirbhar Bharat. Yogi terms move as global approval for PM Modis Aatmanirbhar Bharat. Yogi terms move as global approval for PM Modis Aatmanirbhar Bharat. Yogi terms move as global approval for PM Modis Aatmanirbhar Bharat. Bharat Biotechs Covaxin has got WHO approval on Wednesday as the technical advisory team has recommended the India-made vaccine for emergency use. Bharat Biotechs Covaxin has got WHO approval on Wednesday as the technical advisory team has recommended the India-made vaccine for emergency use. Bharat Biotechs Covaxin has got WHO approval on Wednesday as the technical advisory team has recommended the India-made vaccine for emergency use. Bharat Biotechs Covaxin has got WHO approval on Wednesday as the technical advisory team has recommended the India-made vaccine for emergency use. Covaxin is a whole virion-inactivated vaccine. Covaxin is a whole virion-inactivated vaccine. Covaxin is a whole virion-inactivated vaccine. Covaxin is a whole virion-inactivated vaccine.
The World Health Organization has granted approval for Indian drugmaker Bharat Biotechs home-grown COVID-19 vaccine for emergency use -. The World Health Organization has granted approval for Indian drugmaker Bharat Biotechs home-grown COVID-19 vaccine for emergency use -. The World Health Organization has granted approval for Indian drugmaker Bharat Biotechs home-grown COVID-19 vaccine for emergency use -. The World Health Organization has granted approval for Indian drugmaker Bharat Biotechs home-grown COVID-19 vaccine for emergency use -. Bharat Biotech on Wednesday also issued a statement saying that Central Drugs Standard Control Organisation CDSCO has approved the extension of. Bharat Biotech on Wednesday also issued a statement saying that Central Drugs Standard Control Organisation CDSCO has approved the extension of. Bharat Biotech on Wednesday also issued a statement saying that Central Drugs Standard Control Organisation CDSCO has approved the extension of. Bharat Biotech on Wednesday also issued a statement saying that Central Drugs Standard Control Organisation CDSCO has approved the extension of. Dr Swaminathans remark came after WHO approved Covaxin for emergency use She said Covaxin by no means took the longest to secure WHO approval She said on. Dr Swaminathans remark came after WHO approved Covaxin for emergency use She said Covaxin by no means took the longest to secure WHO approval She said on. Dr Swaminathans remark came after WHO approved Covaxin for emergency use She said Covaxin by no means took the longest to secure WHO approval She said on. Dr Swaminathans remark came after WHO approved Covaxin for emergency use She said Covaxin by no means took the longest to secure WHO approval She said on.
The approval by WHO was expected by the end of October but is still. The approval by WHO was expected by the end of October but is still. The approval by WHO was expected by the end of October but is still. The approval by WHO was expected by the end of October but is still. India News LIVE Sensex Today November Bank Holiday 2021 Dogecoin Price Cryptocurrency Binance Coin Price What are multi asset funds What are balanced mutual funds Global markets Mutual funds Breaking news LTC Cash Voucher Scheme SGX Nifty Sensex Live IRCTC share price Infosys share price Rupee. India News LIVE Sensex Today November Bank Holiday 2021 Dogecoin Price Cryptocurrency Binance Coin Price What are multi asset funds What are balanced mutual funds Global markets Mutual funds Breaking news LTC Cash Voucher Scheme SGX Nifty Sensex Live IRCTC share price Infosys share price Rupee. India News LIVE Sensex Today November Bank Holiday 2021 Dogecoin Price Cryptocurrency Binance Coin Price What are multi asset funds What are balanced mutual funds Global markets Mutual funds Breaking news LTC Cash Voucher Scheme SGX Nifty Sensex Live IRCTC share price Infosys share price Rupee. India News LIVE Sensex Today November Bank Holiday 2021 Dogecoin Price Cryptocurrency Binance Coin Price What are multi asset funds What are balanced mutual funds Global markets Mutual funds Breaking news LTC Cash Voucher Scheme SGX Nifty Sensex Live IRCTC share price Infosys share price Rupee. Home India News General News Covaxin gets WHO nod. Home India News General News Covaxin gets WHO nod. Home India News General News Covaxin gets WHO nod. Home India News General News Covaxin gets WHO nod.

Covaxin Approval Soon Who Chief Scientist Says Phase 3 Trial Data Looks Good Breaking News Youtube
Source Image @ www.youtube.com
Covaxin who approval news today | Covaxin Approval Soon Who Chief Scientist Says Phase 3 Trial Data Looks Good Breaking News Youtube
Collection of Covaxin who approval news today ~ Speaking to NDTV Swaminathan said In this case it should be much faster. Speaking to NDTV Swaminathan said In this case it should be much faster. Speaking to NDTV Swaminathan said In this case it should be much faster. The approval of Covaxin for emergency use listing will amount to recognition and help pave the way for people who have taken the dose to qualify for vaccine mandates in other countries. The approval of Covaxin for emergency use listing will amount to recognition and help pave the way for people who have taken the dose to qualify for vaccine mandates in other countries. The approval of Covaxin for emergency use listing will amount to recognition and help pave the way for people who have taken the dose to qualify for vaccine mandates in other countries. Following the WHOs EUL Listing approval Bharat Biotech MD Dr Krishna Ella said in a video Today Covaxin got EUL from WHO. Following the WHOs EUL Listing approval Bharat Biotech MD Dr Krishna Ella said in a video Today Covaxin got EUL from WHO. Following the WHOs EUL Listing approval Bharat Biotech MD Dr Krishna Ella said in a video Today Covaxin got EUL from WHO.
The approval given by the World Health Organization WHO to Covaxin has opened up the indigenous vaccine for worldwide use Indian Council of. The approval given by the World Health Organization WHO to Covaxin has opened up the indigenous vaccine for worldwide use Indian Council of. The approval given by the World Health Organization WHO to Covaxin has opened up the indigenous vaccine for worldwide use Indian Council of. Explore more on Covaxin Who Approval. Explore more on Covaxin Who Approval. Explore more on Covaxin Who Approval. Covaxin has been approved by many countries around the world including Australia Greece and Sri Lanka among others. Covaxin has been approved by many countries around the world including Australia Greece and Sri Lanka among others. Covaxin has been approved by many countries around the world including Australia Greece and Sri Lanka among others.
Around 10 of the 107 billion doses. Around 10 of the 107 billion doses. Around 10 of the 107 billion doses. In what can be touted as a win for India the Technical Advisory Group of WHO which met today gave its nod for the Emergency Use Listing status for Bharat Biotechs Covaxin. In what can be touted as a win for India the Technical Advisory Group of WHO which met today gave its nod for the Emergency Use Listing status for Bharat Biotechs Covaxin. In what can be touted as a win for India the Technical Advisory Group of WHO which met today gave its nod for the Emergency Use Listing status for Bharat Biotechs Covaxin. The World Health Organisations panel had last week sought additional clarifications from the Hyderabad-based pharma firm. The World Health Organisations panel had last week sought additional clarifications from the Hyderabad-based pharma firm. The World Health Organisations panel had last week sought additional clarifications from the Hyderabad-based pharma firm.
Around 10 of the 107 billion doses administered so far in the country are of Covaxin. Around 10 of the 107 billion doses administered so far in the country are of Covaxin. Around 10 of the 107 billion doses administered so far in the country are of Covaxin. Biocon Chairperson Kiran Mazumdar Shaw on Wednesday November 3 told India Today TV The fact that Covaxin has been approved by WHO is good validation for Indian innovation She said it. Biocon Chairperson Kiran Mazumdar Shaw on Wednesday November 3 told India Today TV The fact that Covaxin has been approved by WHO is good validation for Indian innovation She said it. Biocon Chairperson Kiran Mazumdar Shaw on Wednesday November 3 told India Today TV The fact that Covaxin has been approved by WHO is good validation for Indian innovation She said it. The WHO today issued an emergency use listing EUL for Covaxin adding to a growing portfolio of vaccines validated by the UN health agency for the prevention of. The WHO today issued an emergency use listing EUL for Covaxin adding to a growing portfolio of vaccines validated by the UN health agency for the prevention of. The WHO today issued an emergency use listing EUL for Covaxin adding to a growing portfolio of vaccines validated by the UN health agency for the prevention of.
The World Health Organisation WHO on Wednesday approved India-made Covid-19 vaccine developed by Bharat Biotech. The World Health Organisation WHO on Wednesday approved India-made Covid-19 vaccine developed by Bharat Biotech. The World Health Organisation WHO on Wednesday approved India-made Covid-19 vaccine developed by Bharat Biotech. 4th November 2021 0034 IST Covaxin Gets WHO Nod. 4th November 2021 0034 IST Covaxin Gets WHO Nod. 4th November 2021 0034 IST Covaxin Gets WHO Nod. Covaxin was approved for emergency use in India on January 3. Covaxin was approved for emergency use in India on January 3. Covaxin was approved for emergency use in India on January 3.
The final approval has come after a meeting of the global health bodys technical advisory group today Nov. The final approval has come after a meeting of the global health bodys technical advisory group today Nov. The final approval has come after a meeting of the global health bodys technical advisory group today Nov. 3 following a thorough risk-benefit analysis of adding Covaxin to the global vaccine. 3 following a thorough risk-benefit analysis of adding Covaxin to the global vaccine. 3 following a thorough risk-benefit analysis of adding Covaxin to the global vaccine. Its a momentous occasion for Indian innovation entrepreneurship. Its a momentous occasion for Indian innovation entrepreneurship. Its a momentous occasion for Indian innovation entrepreneurship.
The technical advisory group which gives licence to a vaccine for its emergency use listing EUL had asked Bharat Biotech on October 26 for additional data. The technical advisory group which gives licence to a vaccine for its emergency use listing EUL had asked Bharat Biotech on October 26 for additional data. The technical advisory group which gives licence to a vaccine for its emergency use listing EUL had asked Bharat Biotech on October 26 for additional data. Covaxin is made by Hyderabad-based Bharat Biotech FileHighlightsCovaxin has been cleared for use in all age groups 18 However no recommendation News National. Covaxin is made by Hyderabad-based Bharat Biotech FileHighlightsCovaxin has been cleared for use in all age groups 18 However no recommendation News National. Covaxin is made by Hyderabad-based Bharat Biotech FileHighlightsCovaxin has been cleared for use in all age groups 18 However no recommendation News National. The WHO on Wednesday granted approval for Emergency Use Listing EUL for Bharat Biotechs COVID-19 vaccine Covaxin. The WHO on Wednesday granted approval for Emergency Use Listing EUL for Bharat Biotechs COVID-19 vaccine Covaxin. The WHO on Wednesday granted approval for Emergency Use Listing EUL for Bharat Biotechs COVID-19 vaccine Covaxin.
File Photo File Photo Australia Monday recognised Indias Covaxin for the purpose of travel to the country as it eased curbs on international travel adding to the list of many other nations which have given their nod to Bharat Biotechs Covid-19 vaccine. File Photo File Photo Australia Monday recognised Indias Covaxin for the purpose of travel to the country as it eased curbs on international travel adding to the list of many other nations which have given their nod to Bharat Biotechs Covid-19 vaccine. File Photo File Photo Australia Monday recognised Indias Covaxin for the purpose of travel to the country as it eased curbs on international travel adding to the list of many other nations which have given their nod to Bharat Biotechs Covid-19 vaccine. The Technical Advisory Group of WHO which met today has recommended the Emergency Use Listing status for Bharat Biotechs Covaxin - a decision that will ease international travel and the export of the indigenous vaccine. The Technical Advisory Group of WHO which met today has recommended the Emergency Use Listing status for Bharat Biotechs Covaxin - a decision that will ease international travel and the export of the indigenous vaccine. The Technical Advisory Group of WHO which met today has recommended the Emergency Use Listing status for Bharat Biotechs Covaxin - a decision that will ease international travel and the export of the indigenous vaccine. Travellers who have taken both doses of Bharat Biotechs Covid-19 vaccine Covaxin will be allowed to enter the US from November 8 new agency ANI reported. Travellers who have taken both doses of Bharat Biotechs Covid-19 vaccine Covaxin will be allowed to enter the US from November 8 new agency ANI reported. Travellers who have taken both doses of Bharat Biotechs Covid-19 vaccine Covaxin will be allowed to enter the US from November 8 new agency ANI reported.
The advisory committee is scheduled to meet today and the approval for Covaxin is expected to be announced after the meeting. The advisory committee is scheduled to meet today and the approval for Covaxin is expected to be announced after the meeting. The advisory committee is scheduled to meet today and the approval for Covaxin is expected to be announced after the meeting. Yogi Terms Move As Global Approval For PM Modis Aatmanirbhar Bharat With the World Health Organisation WHO approving Bharat Biotechs COVID-19 vaccine Covaxin for emergency use Yogi. Yogi Terms Move As Global Approval For PM Modis Aatmanirbhar Bharat With the World Health Organisation WHO approving Bharat Biotechs COVID-19 vaccine Covaxin for emergency use Yogi. Yogi Terms Move As Global Approval For PM Modis Aatmanirbhar Bharat With the World Health Organisation WHO approving Bharat Biotechs COVID-19 vaccine Covaxin for emergency use Yogi. WHOs approval for Covaxin will facilitate process of India helping. WHOs approval for Covaxin will facilitate process of India helping. WHOs approval for Covaxin will facilitate process of India helping.
Yogi terms move as global approval for PM Modis Aatmanirbhar Bharat. Yogi terms move as global approval for PM Modis Aatmanirbhar Bharat. Yogi terms move as global approval for PM Modis Aatmanirbhar Bharat. Bharat Biotechs Covaxin has got WHO approval on Wednesday as the technical advisory team has recommended the India-made vaccine for emergency use. Bharat Biotechs Covaxin has got WHO approval on Wednesday as the technical advisory team has recommended the India-made vaccine for emergency use. Bharat Biotechs Covaxin has got WHO approval on Wednesday as the technical advisory team has recommended the India-made vaccine for emergency use. Covaxin is a whole virion-inactivated vaccine. Covaxin is a whole virion-inactivated vaccine. Covaxin is a whole virion-inactivated vaccine.
The World Health Organization has granted approval for Indian drugmaker Bharat Biotechs home-grown COVID-19 vaccine for emergency use -. The World Health Organization has granted approval for Indian drugmaker Bharat Biotechs home-grown COVID-19 vaccine for emergency use -. The World Health Organization has granted approval for Indian drugmaker Bharat Biotechs home-grown COVID-19 vaccine for emergency use -. Bharat Biotech on Wednesday also issued a statement saying that Central Drugs Standard Control Organisation CDSCO has approved the extension of. Bharat Biotech on Wednesday also issued a statement saying that Central Drugs Standard Control Organisation CDSCO has approved the extension of. Bharat Biotech on Wednesday also issued a statement saying that Central Drugs Standard Control Organisation CDSCO has approved the extension of. Dr Swaminathans remark came after WHO approved Covaxin for emergency use She said Covaxin by no means took the longest to secure WHO approval She said on. Dr Swaminathans remark came after WHO approved Covaxin for emergency use She said Covaxin by no means took the longest to secure WHO approval She said on. Dr Swaminathans remark came after WHO approved Covaxin for emergency use She said Covaxin by no means took the longest to secure WHO approval She said on.
The approval by WHO was expected by the end of October but is still. The approval by WHO was expected by the end of October but is still. The approval by WHO was expected by the end of October but is still.

No Emergency Use Approval For Bharat Biotech
Source Image @ www.india.com
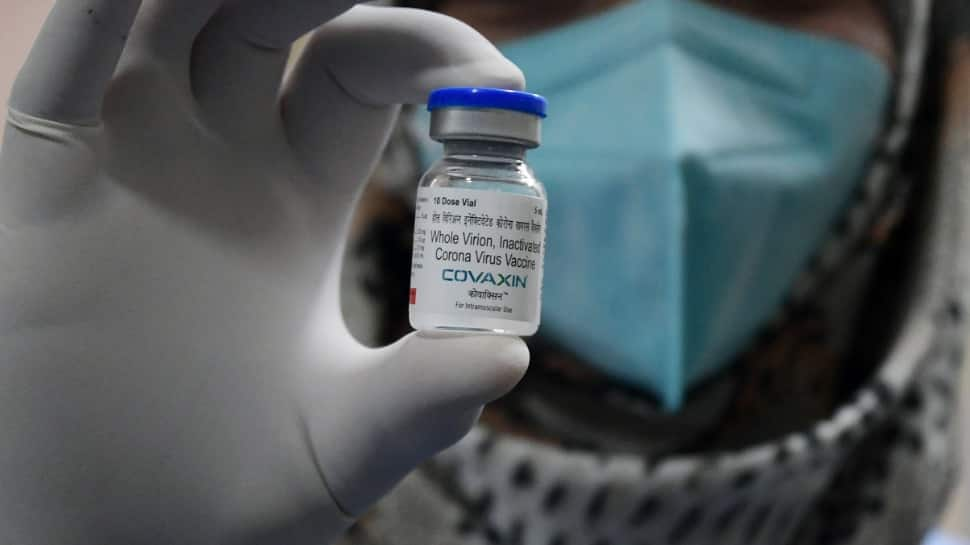
Who Seeks Additional Clarifications From Bharat Biotech For Covaxin S Emergency Use Approval India News Zee News
Source Image @ zeenews.india.com
Covaxin S Lack Of Usfda Approval A Roadblock In Getting Who Green Flag The Hindu Businessline
Source Image @ www.thehindubusinessline.com
)
Decision On Clearance For Covaxin In October World Health Organisation
Source Image @ www.ndtv.com